Best seller
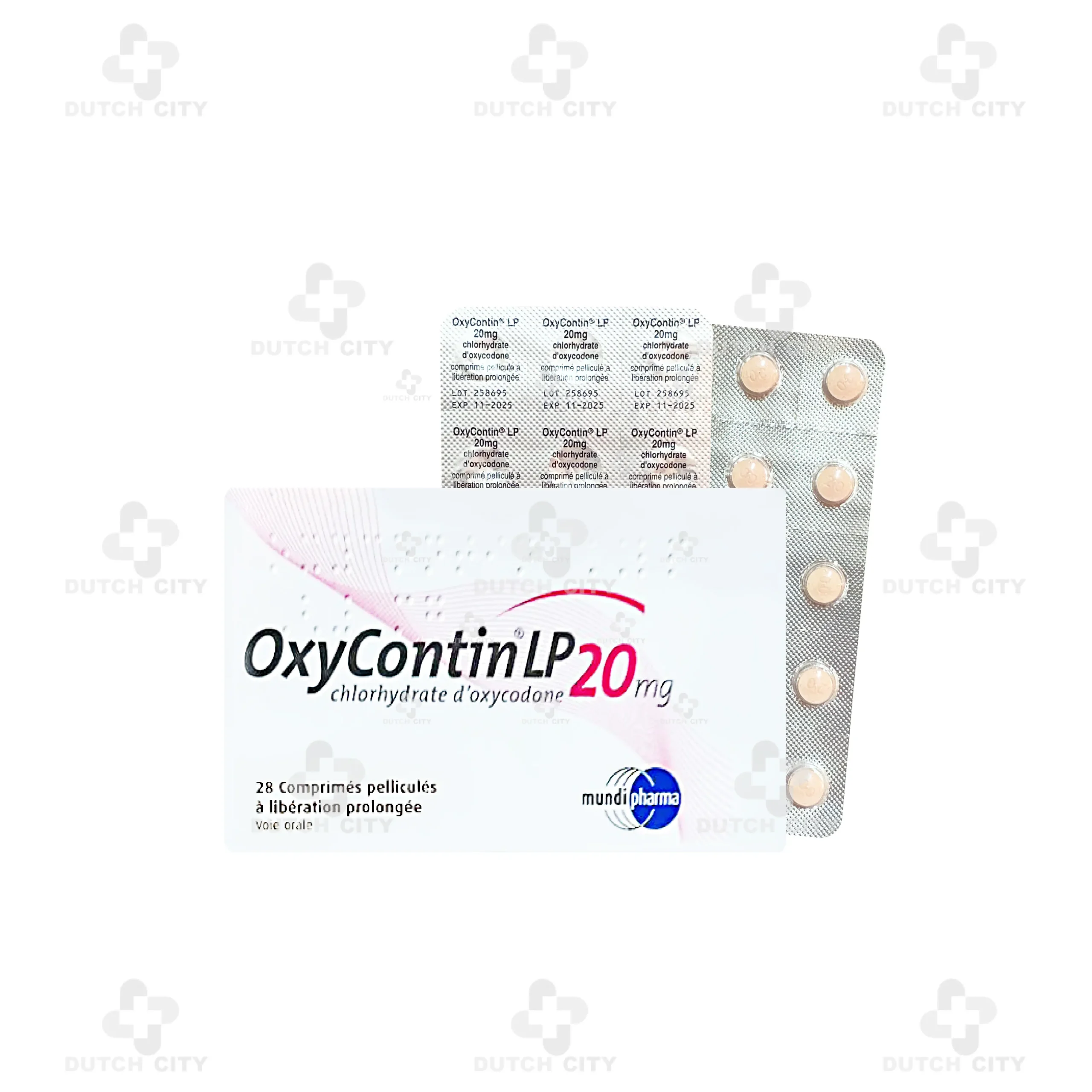
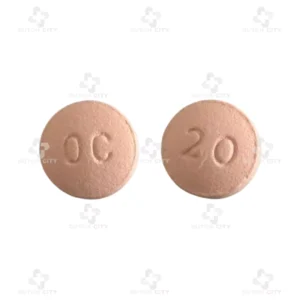
OxyContin® 20mg (Mundipharma Company Uk 🇬🇧)
€200 – €1,360
OxyContin

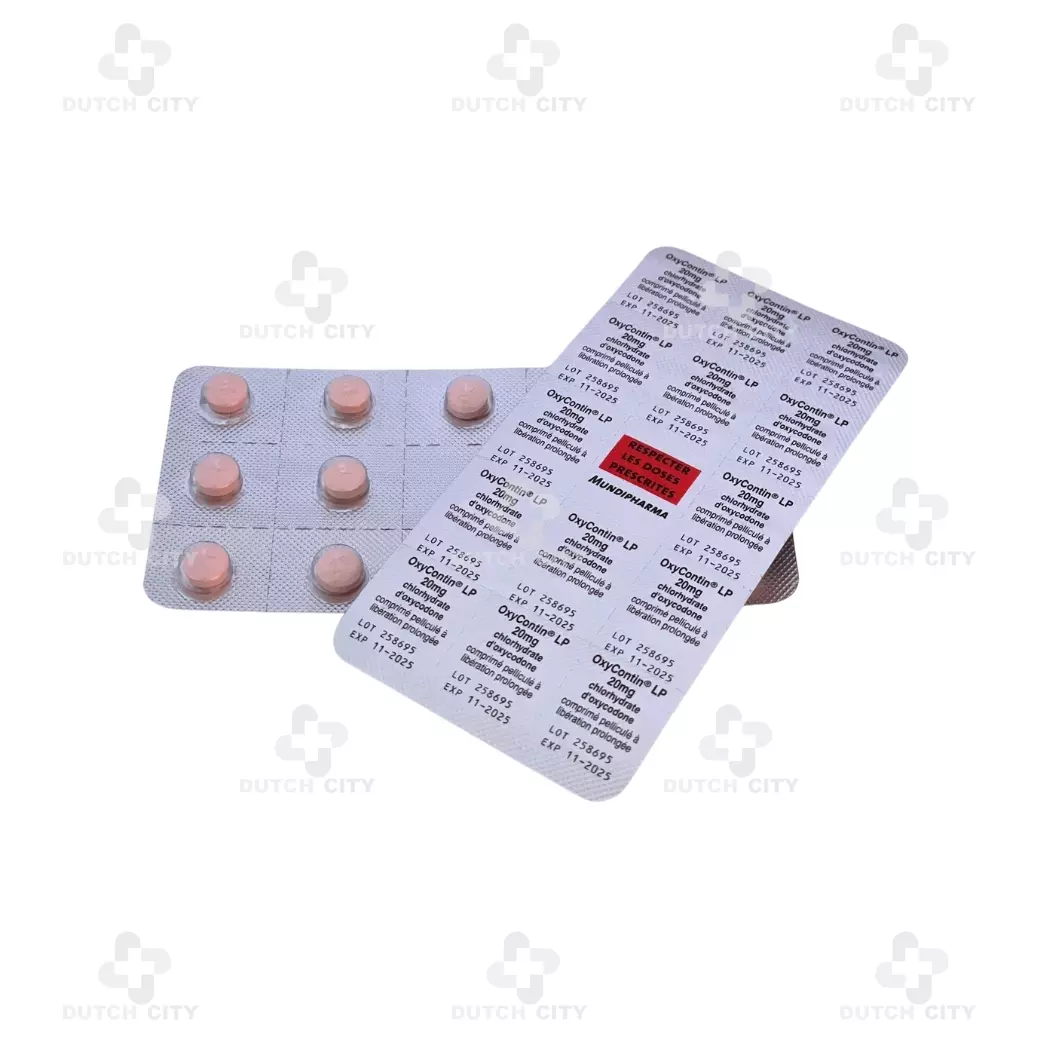
(1 box – 2 strips or 28 tablets)
Oxycodone is the ultimate pain reliever. It’s a drug that works for a long time and when you wake up it’s still not cured. The medicine has the best effect and taking it with Lean is the best.
Product Name and Active Ingredient
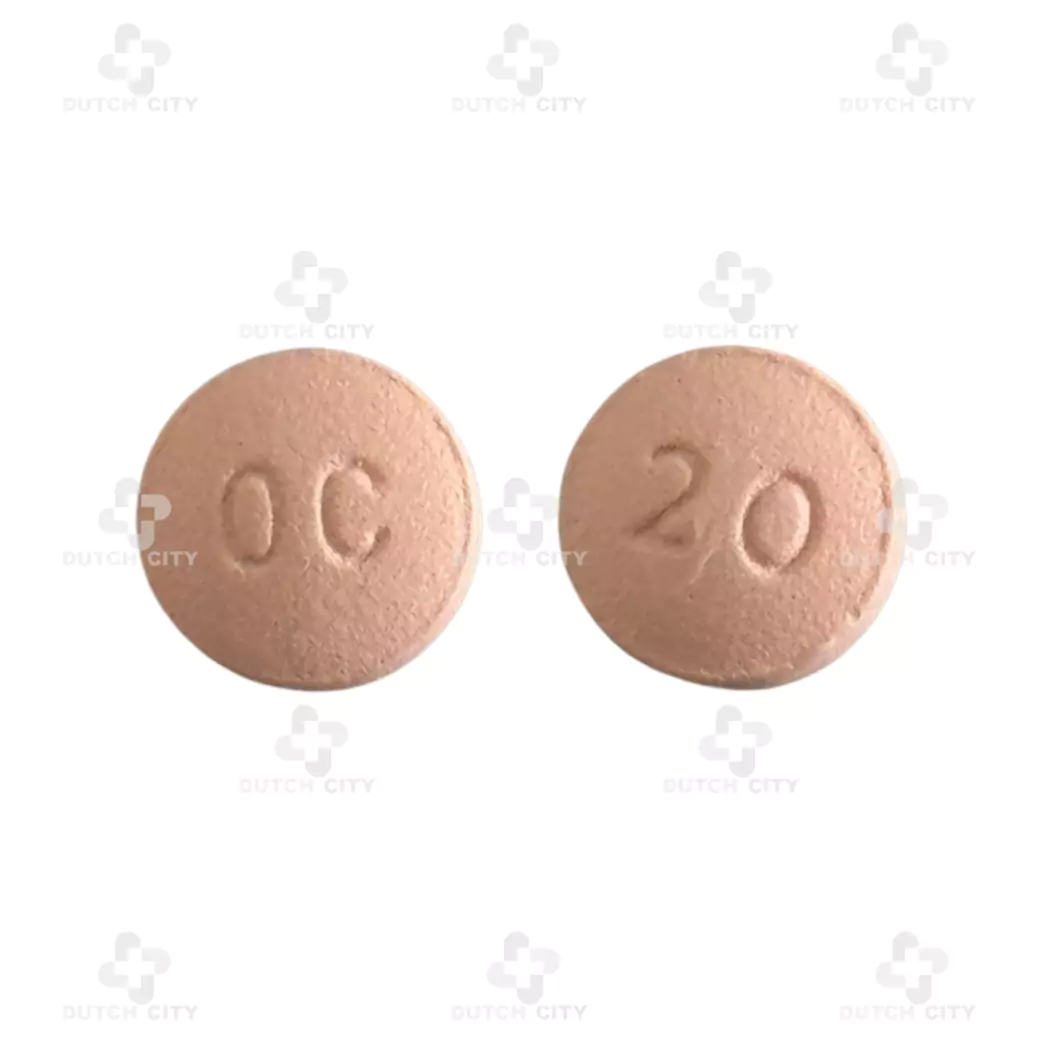
Brand Name:OxyContin® 20 mg
Generic Name:Oxycodone Hydrochloride
Drug Class:Opioid Analgesic
Dosage Form:Prolonged-Release Tablet (12-hour extended release)
Composition
Active Ingredient:Each tablet contains 20 mg of oxycodone hydrochloride (equivalent to 20 mg of oxycodone base)
Manufacturer and Country of Origin
Manufacturer: Mundipharma Pharmaceuticals Ltd. (previously Napp Pharmaceuticals)
Country of Origin: Ireland (EU)
– 1 Strip – €200 !!
– 2 Strips – €420 (210)
– 3 Strips – €600 (200)
– 6 Strip – €1140 (190)
– 8 Strip – €1360 (170)‼️
– 2 Strips – €420 (210)
– 3 Strips – €600 (200)
– 6 Strip – €1140 (190)
– 8 Strip – €1360 (170)‼️
2 Strips or 28 Tablets per 1 Box
#Oxy #Oxi #Perc #Percocet #Oxycodone #Morphine #Codeine #Dihydrocodeine #DHC #Dihydrocodein









Reviews
There are no reviews yet.